Presenter:
Xianwen Mao
Abstract:
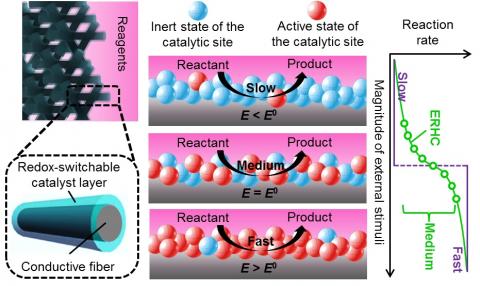
We report a method to control reaction kinetics using electrochemically responsive heterogeneous catalysis (ERHC). An ERHC system should possess a hybrid structure composed of an electron-conducting porous framework coated with redox-switchable catalysts. In contrast to other types of responsive catalysis, ERHC combines all the following desired characteristics for a catalysis control strategy: continuous variation of reaction rates as a function of the magnitude of external stimulus, easy integration into fixed-bed flow reactors, and precise spatial and temporal control of the catalyst activity. Herein we demonstrate a facile approach to fabricating a model ERHC system that consists of carbon microfibers with conformal redox polymer coating. Second, using a Michael reaction whose kinetics depends on the redox state of the redox polymer catalyst, we show that use of different electrochemical potentials permits continuous adjustment of the reaction rates. The dependence of the reaction rate on the electrochemical potential generally agrees with the Nernstian prediction, with minor discrepancies due to the multi-layer nature of the polymer film. Additionally, we show that the ERHC system can be employed to manipulate the shape of the reactant concentration – time profile in a batch reactor through applying customized potential – time programs. Furthermore, we performed COMSOL simulation for an ERHC-integrated flow reactor, demonstrating highly flexible manipulation of reactant concentrations as a function of both location and time.