Presenter:
Jonathan C. Barnes
Abstract:
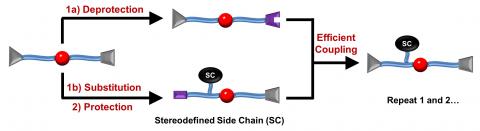
Nature relies on macromolecules with perfectly defined length, sequence, and chirality to achieve an array of functions. Chemists have long sought to emulate Nature’s macromolecules, but absolute control over structure remains a key challenge for macromolecular synthesis. In an attempt to gain control over these features, we report a novel iterative exponential growth plus side-chain functionalization (IEG+) process that facilitates the efficient synthesis of oligotriazoles with uniform length, sequence, and stereoconfiguration. IEG+ begins from a key monomer that features an enantiopure epoxide and a silyl-protected alkyne. Epoxide opening with azide anion exposes a new hydroxyl group that serves as a handle for incorporation of new side-chain functionality. Coupling of this azide with fluoride-deprotected monomer generates a new first-generation “dimer” that can be re-subjected to cycles of IEG+. Since new functionality can be introduced with each IEG+ cycle, the products of n cycles are triazoligomers with 2^n length, stereodefined backbones, and variable side-chain sequences. We anticipate that these new macromolecules and the IEG+ strategy will find broad application in macromolecular science.