Presenter:
Yiran Zheng
Abstract:
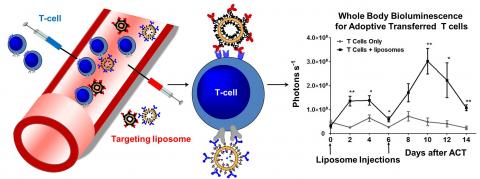
In adoptive cell therapy (ACT), autologous tumor-specific T-cells isolated from cancer patients are activated and expanded ex vivo before infusing back into the individual to eliminate metastatic tumors. A limitation of this promising approach in solid tumors is the loss of ACT T-cell effector function in vivo due to the highly immunosuppressive tumor microenvironment. Systemic administration of supporting adjuvant drugs such as interleukins, chemotherapy and other immunomodulators are often accompanied by serious toxicities and may still fail to optimally stimulate ACT cells. Here we propose a novel strategy to repeatedly stimulate ACT T-cells, using cytokines or ACT-cell-specific antibodies (Ab) as ligands to target drug-loaded PEGylated nanoparticles (NP) to transferred T-cells in vivo. Injected NPs bind to T cells in situ to provide adjuvant drugs for T cell expansion, replacing repeated laborious T cell transfers with simple injections of NPs. Two types of targeting ligands were tested: 1) Abs against innocuous congenic markers on ACT cells and 2) immunostimulatory cytokines that can synergize co-stimulation with targeted delivery of drugs. Using F(ab’)2 fragments against Thy1.1 or an engineered interleukin-2 (IL-2) molecule on an Fc framework as targeting ligands, we demonstrated that >95% of ACT cells can be targeted with liposomes following a single injection in vivo. IL-2-conjugated liposomes both target ACT cells and induce repeated waves of ACT T-cell proliferation and tumor regression in B16F10 melanoma tumors. Currently, we are testing the efficacy of using our targeting liposomes to deliver encapsulated immune-suppression blocking drugs or incorporated Toll Like Receptor 2 agonist Pam3Cys to ACT T cells in tumor therapy. These results demonstrate the feasibility of repeated functional targeting of T-cells in vivo for specific delivery of immunomodulators in ACT regimens.